NEXT GENERATION THERAPY FOR STROKE RECOVERY: NIH GRANT ADVANCES WIRELESS VNS DEVICE TESTING
Bioengineering professors at The University of Texas at Dallas recently earned a $1.4 million grant from the National Institutes of Health to study the use of wireless vagus nerve stimulation (VNS) to enhance recovery after stroke.
Dr. Seth Hays, assistant professor of bioengineering in the Erik Jonsson School of Engineering and Computer Science and fellow, Eugene McDermott professor, and Dr. Robert Rennaker, professor of bioengineering, professor of neuroscience, Texas Instruments Distinguished Chair in Bioengineering and associate director of the Texas Biomedical Device Center have created a new wireless, low-cost clinical grade device to eliminate the challenges of previous devices. The grant will provide funding to complete testing so the new devices can meet regulatory standards and continue to human studies.
“This is the first human-grade implant designed, developed and manufactured at a university,” Rennaker said.
The National Institutes of Health awards funding depending upon the stage of research and emphasizes projects of “high scientific caliber that are relevant to public health needs,” according to their website. The funding for this project will allow for final verification and validation of the devices, which will move them to clinical trials necessary for FDA approval.
Stroke is a common, debilitating condition. Hays, the American Heart Association Robert G. Siekert New Investigator for Stroke in 2015 and principal investigator of this grant, has previously studied the use of VNS for stroke and found that a combination of the stimulation therapy plus rehabilitation may enhance recovery.
Improving Wireless VNS Device Accessibility and Production
The use of vagus nerve stimulation paired with rehabilitation was invented at UT Dallas by Dr. Michael Kilgard, professor of neuroscience, Margaret Fonde Jonsson Professor and executive director of the Texas Biomedical Device Center. Hays, Rennaker and Kilgard are now developing VNS for several types of notoriously treatment resistant neurological injury, including stroke, brain hemorrhage, traumatic brain injury and spinal cord injury. However, VNS is currently limited by the fragility, large size and high cost of the devices themselves. For stroke patients, who need to have regular MRIs, the original devices can also interfere with their treatment.

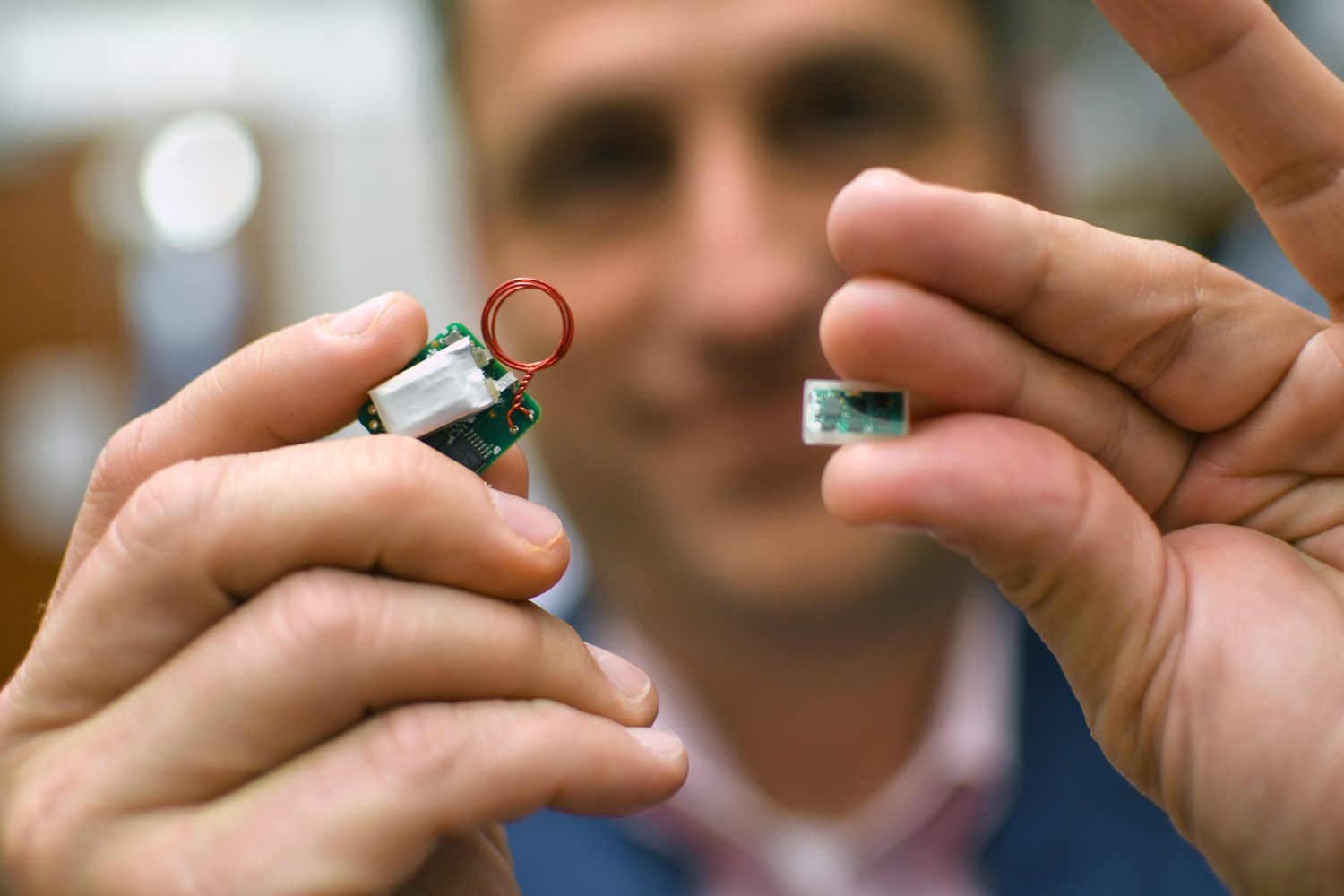
“The original devices are big, and you can’t use them for a traditional MRI,” Hays said. “We got rid of the leads and battery, then made them 50 times smaller.”
“And 10 times less expensive,” Rennaker said.
The new production method is cheaper and can be produced entirely through automated processes without human contact. The devices are created with a wafer and hermetically sealed between two layers of glass for protection. Local company Zyvex Labs lasered, welded and cut the glass plates.
After VNS receives FDA approval, the new wireless devices are expected to expand both access and ease of use, particularly because the new devices are compatible with traditional MRIs. Additionally, patients will be able to use the VNS remotely when they complete rehabilitation exercises at home, using a mobile app with wireless connectivity.
“The stimulation enhances the patient’s neuroplasticity, which can make the combined stimulation and rehabilitation three times more effective over traditional rehabilitation alone,” Hays said. “The key is doing both at the same time. This is a fundamentally different approach – giving the brain the boost it needs to rewire itself, capitalizing on a similar approach as vaccine technology.”
University-Driven Innovation
As the wireless VNS device has been developed entirely at UT Dallas, the process has provided numerous learning experiences for bioengineering students. According to Rennaker, who has worked on applying the technology to other conditions including tinnitus and post-traumatic stress disorder, more than dozens of undergraduate and graduate papers are guiding the technology.
“We have student workers learning the process of FDA trials,” Rennaker said. “Over 500 students have been trained in the laboratory, and it currently supports 12 PhD candidates and postdoctoral fellows. Each student is working on some aspect of the project. It’s been a unique, collective effort.”
Throughout the process, the team has worked with physicians including Dr. Jane Wigginton, Dr. Teresa Chan and Dr. Ted Mau at UT Southwestern Medical Center, as well as rehabilitation therapists at Baylor Scott and White Health. Additionally, the team acknowledges the project has been spurred forward by this and other significant NIH grants.
“We are grateful for the support of the National Institutes of Health for the funded research project,” Rennaker said. “We also must thank the Communities Foundation of Texas for the W.W. Caruth, Jr. Fund Grant which allowed us to develop the original prototype.”
The combined grant funding and student research efforts toward the project is leading to cost-effective innovation that researchers hope will have innumerable benefits to patients suffering from brain injury due to stroke.
“It has cost around $6 million to complete the research at UT Dallas, as compared to $30 million in a typical commercial research setting,” Rennaker said. “As a Tier 1 Research Institution, we now have the tools to carry this project to completion.”
A version of this story also ran in News Center.
Contact
Laura Schmidt
972-883-2158
laura.schmidt@utdallas.edu




